Donating skin to create personalized stem cells
In this post, I’ll review my recent experience donating skin cells in order to have my very own line of induced pluripotent stem cells (iPSC) created for research. First, I’ll provide a bit of background on what iPSC are, and why they’re useful.
From the researcher perspective
Induced pluripotent stem cells, or iPSC, are adult human cells that have been genetically transformed from a specific adult cell type (for example, skin cells) to a “pluripotent” stem cell state. “Pluripotent” means the cells can become anything, much like cells in an embryo haven’t yet decided whether to become skin, or brain, or heart, or any other type of cell. When scientists “reprogram” skin cells, the cells travel backwards in developmental time to a moment at which their cell fate is yet to be determined. And from there, according to a rapidly growing set of protocols, they can be “differentiated” into a particular cell type of interest. This makes it possible to study human neurons in a way that has never been possible before.

Above, human induced pluripotent stem cells have been differentiated into NPCs, or neural progenitor cells — an intermediate stage on the way to a neuronal fate.
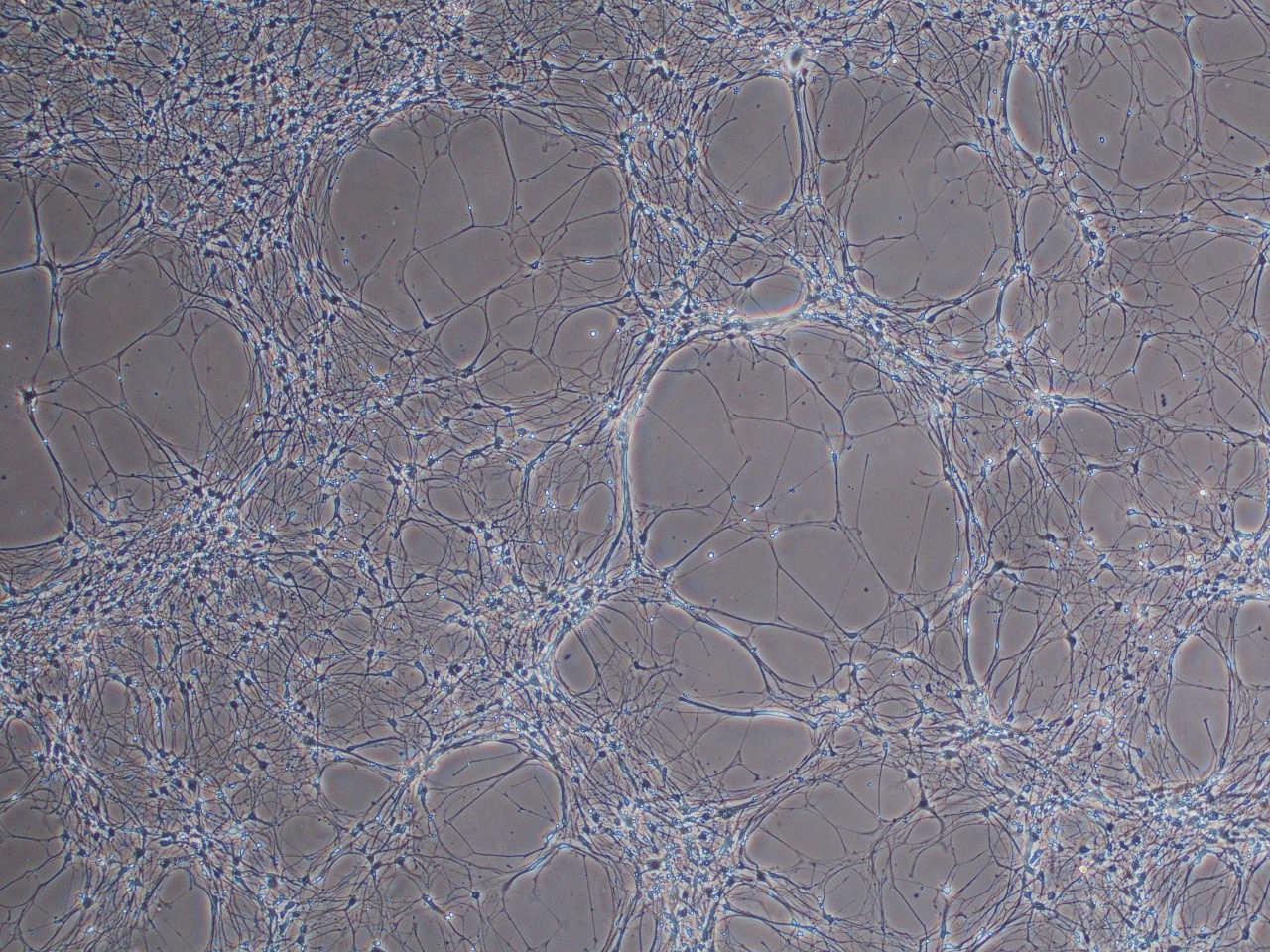
From NPCs, these cells have been differentiated into human neurons in a dish.
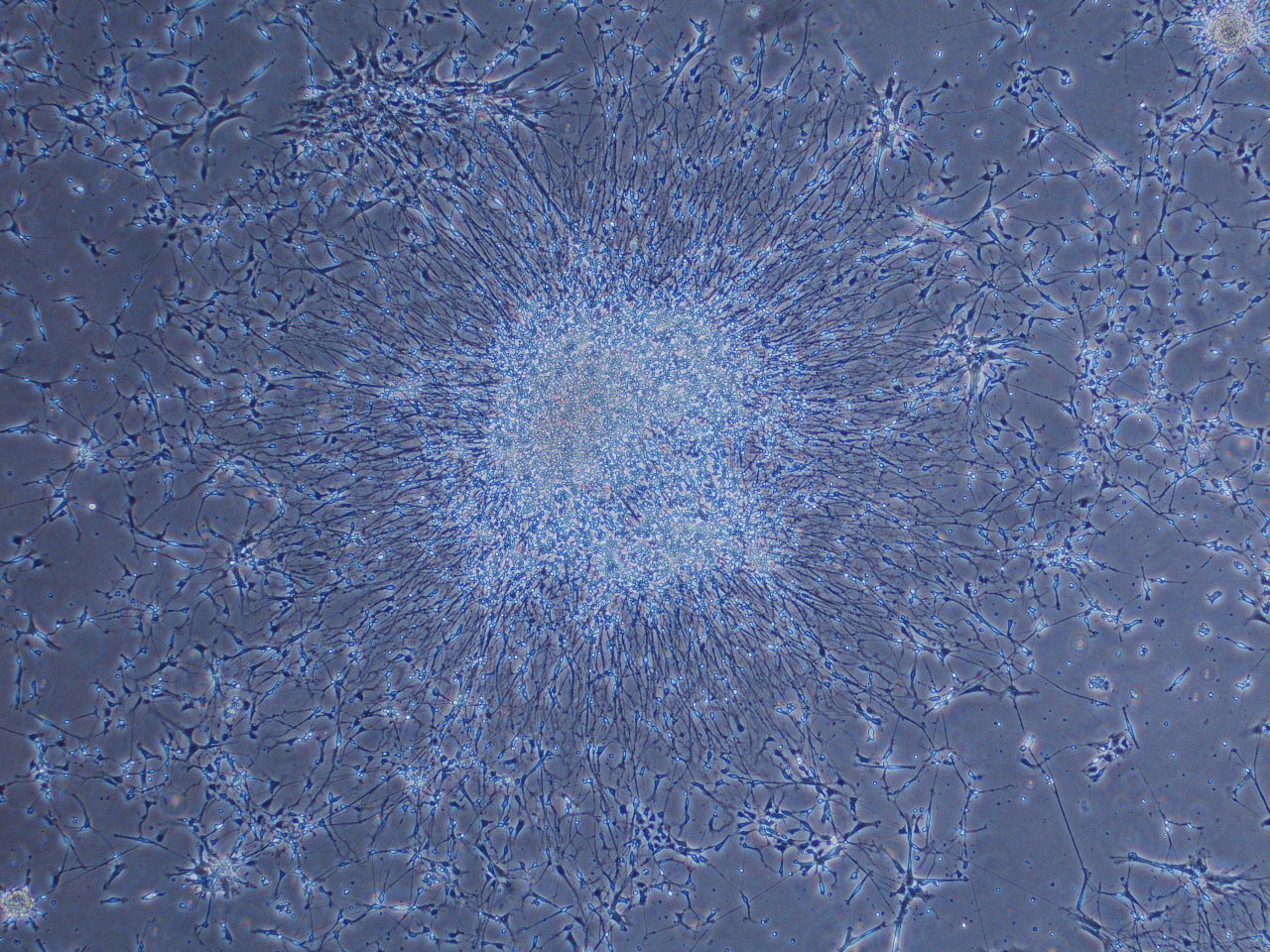
Sometimes, neurons-in-a-dish look like this! Crazy.
iPSC have, in the past several years, generated a huge amount of excitement as tools in human disease research. The breakthrough iPSC paper that first demonstrated “reprogramming” of adult human cells to stem cells was published in 2006, and for this work author Shinya Yamanaka shared the Nobel Prize for Physiology or Medicine in 2012. Yamanaka and colleagues started from twenty-four genes thought to be important for pluripotency, and whittled them down to a core team of four essential genes that can be “added” to an adult cell in order to turn it back into a stem cell.
Today, these “Yamanaka factors” have been used to create hundreds of iPSC lines. iPSC can now be created from a variety adult cell types, and according to a variety of protocols. For more detail on the original Yamanaka paper and the work that has followed it, see this blog post on CureFFI.org.
So why the excitement – how are iPSC useful, and how might they be even more useful in the future? Three broad potential uses are generally cited for iPSC. First, they can serve as a human disease model, allowing for in vitro observation of disease processes that are difficult to observe in real time when they’re happening inside the human body. This provides a particular advantage for hard-to-access tissues like the brain. Second, they may be able to serve as a screening platform for drugs. If we can identify signatures of disease in iPS cells that have a certain disease-associated genetic mutation, then compounds can be screened in a high-throughput manner for their ability to “correct” this signature. Thirdly, and most futuristically speaking, perhaps iPSC could someday serve as therapeutics, for example for own-tissue transplants.
These visions are at varying stages of realization. In research, iPSC are being actively explored as models for dozens of diseases. They are expensive and labor-intensive to maintain, but scores of labs have invested in iPSC-specific facilities. The quest to identify disease signatures in cells is complex but ongoing. Differentiation protocols have proven tricky to standardize, but many smart people are putting their minds to this problem. In recognition of the potential of this technology, researchers in numerous different disease fields, including the prion field, are moving to create banks of iPS cell lines from patients.
I’m pretty familiar with the day-to-day of iPSC because for the past year, I’ve been responsible for a number of iPSC lines that my lab at the Center for Human Genetic Research at Mass General Hospital is using to study Huntington’s Disease. HD is caused by a repeat expansion mutation, and the severity and onset of disease track with the size of the expansion. Our lab looks at lines with various repeat lengths in parallel, in order to assess traits of the cells that are repeat-length dependent.
When I say the cells are a lot of work, I speak from the experience of trying to catch a cab home from the lab at 2am on a Saturday night (not easy, at bar time in Boston!) because the cells needed attention. In general, they can’t go longer than a day without being looked at under a microscope by someone who understands their morphology. Pluripotency isn’t “supposed” to last forever, and if any of their growth conditions are off, the cells are only too happy to randomly differentiate into a mess of different cell types, at which point they’re no longer useful for research. My iPS cells have taught me a lot and are an amazing tool, but there is no relief like the relief I felt in December when I froze them all down for the holidays!

This is how I spend a lot of my time. The iPS room is a harsh mistress.
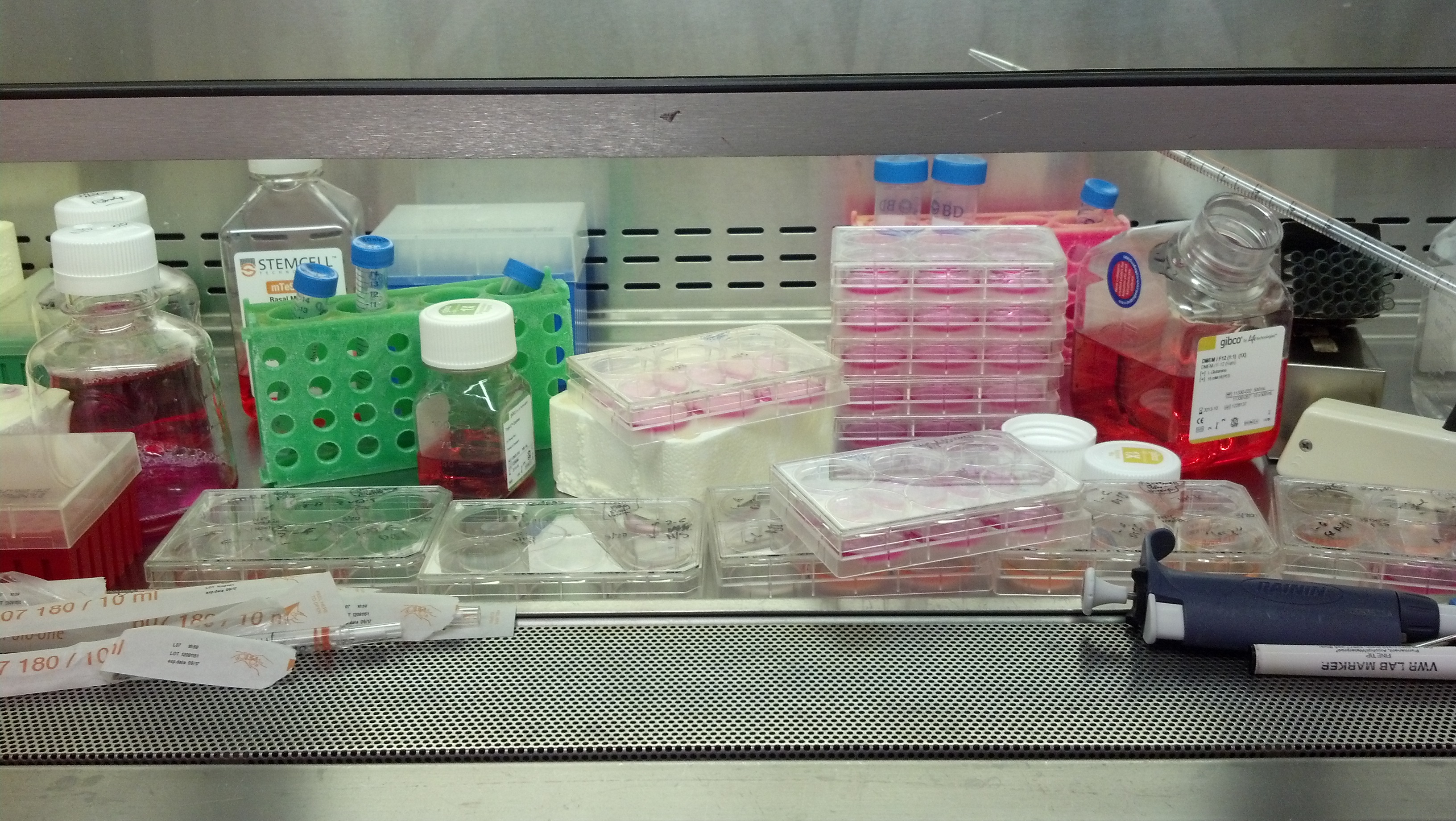
It was that sort of day.
From the patient perspective
Eric and I have been interested in having iPSC made from my cells for more than a year, and finally learned of an opportunity to do so through Dr. Wenquan Zou at Case Western Reserve University. In collaboration with other researchers, Dr. Zou is aiming to create a repository of iPSC lines that have different prion disease mutations, in addition to several control lines. Since I have the mutation that causes fatal familial insomnia, I was eligible to participate.
After speaking with Dr. Zou, I signed a consent form for study participation. This form gave a bit of background on iPSC, and explained what would be required of me for the skin punch procedure and follow-up. It gave an overview of the risks (discomfort from administration of the anesthetic, pain or itching after the anesthetic wears off, possible allergic reactions, emotional discomfort) and benefits (indirect – the advancement of science) of participation. It conveyed to me that I was free to choose not to participate, and could choose to revoke consent at any time up to the creation of iPSC. It also made clear that once the iPSC were created, I would not be able to control their fate – not which institutions were able to work with them, and not the nature of that work (some of which likely can’t even be predicted today.) In addition, I agreed to forfeit commercial interest in the cells. These risks all seemed tolerably mild and/or remote compared to the potential scientific benefit of creating the cell line, and the terms of the contract all seemed reasonable to me.
Once I was enrolled, we set about trying to find a dermatologist in Boston who would be able to do a skin biopsy, and then ship my tissue sample to Dr. Zou’s lab in Cleveland. The search for a doctor wasn’t simple — at least in the Boston area, quoted prices for this procedure varied almost fivefold. We were rewarded for canvassing the options when we ultimately did locate a dermatologist at a local university hospital able to meet the study budget of $175. My skin punch was scheduled for early in the week – a Tuesday morning – to ensure that the sample, after being shipped overnight, would be received on a weekday.
An interesting aspect of the scheduling process is that the local dermatologist, who I’ll refer to as Dr. W., at first expressed willingness to take a skin biopsy for a research study, but dithered when he realized that the focus of the research study was prion diseases. There is a lot of slippage in the way the terms “infectious” and “transmissible” are applied to prion diseases in medical dialogue, and the word “prion” is uniquely able to raise a sort of nebulous alarm in medical professionals. It’s important for PRNP mutation carriers to be aware of these misperceptions, and able to correct them.
In the cases of specific procedures, such as brain surgery, that bring implements into contact with the central nervous system of a patient, it is certainly important for doctors to be aware of best practices. See, for example, the recent incident in New Hampshire. But for most peripheral tissues (i.e. outside of the brain and spinal cord), no infectivity can be detected at all – even in patients with full-blown symptomatic prion disease [WHO 1999]. Meanwhile, patients with a genetic mutation are healthy for decades before disease strikes, and there’s no reason to think these patients are infectious at all while they are still healthy [WHO 1999]. All told, there is no evidence at all that peripheral tissue of an asymptomatic prion protein mutation carrier poses any threat or requires special precautions. The appointment was scheduled once this distinction was clarified.
Upon arrival at the office, I was told that my appointment would last a half hour. I met Dr. W., and we briefly discussed the procedure. He showed me the box of supplies that had been sent to him from the Case Western lab a few days prior. It contained a 15mL plastic Falcon tube with several mL of media into which he would immerse my tissue sample for transport, and cold packs that would keep the sample cool and upright in the box during shipping.
I mentioned to Dr. W. at this point that my skin is prone to scarring, and he recommended that we proceed with a 3mm-diameter biopsy. 4mm is typical, but he called Dr. Zou at Case Western to confirm that 3mm would be sufficient.
Skin biopsies for the creation of iPSC can be taken from various areas of the body; I’ve heard of samples being taken from the elbow, forearm, abdomen, thigh and flank. To minimize discomfort, bleeding and visibility, Dr. W. gave me his recommendation that we choose a spot on my lower hip that wouldn’t be visible even in a swimsuit. I donned a very stylish paper gown, and together we chose a discreet spot on my lower right hip, and he marked it with a pen.
I lay on my side on the patient table, and Dr. W. sterilized the site with alcohol and prepared a shot of the local anesthetic lidocaine. This is the numbing agent typically used in minor surgeries or dental procedures. The formulation contains epinephrine, which constricts arteries with the effect of reducing bleeding. Epinephrine also delays the resorption of lidocaine, which functions to prolong the anesthetic effect.
Now we come to the only part of the procedure that hurt at all. Dr. W. talked me through the injection of lidocaine into our chosen site, and I experienced the sting of the needle followed by several seconds of an acute burning sensation. With the forewarning it was not bad – perhaps on par with a bee sting. Within several seconds, it was over, and I could feel a curious lack of sensation setting in.
At this point Dr. W. left the room for about fifteen minutes to wait for the epinephrine to take effect. I stayed on my side on the table. When he returned, he probed my skin at the intended site and asked if I could feel anything. The truthful answer is that I couldn’t; there was a faint sensation of pressure, but I had no idea what he was poking me with, or even specifically where.
What I know about what follows, I know from what Dr. W. narrated and what I have read about skin biopsy protocols. Lying on my side, looking straight ahead, I had no awareness at all of the insertion of the skin punch, which “cores” the small designated amount of tissue. I understand that he then stabilized the selected tissue with a forceps, and used a scalpel to cleanly remove it. Finally, he sewed the wound together with one stitch, made with absorbable fiber so that I wouldn’t have to return to the office to have it removed. Even this one stitch, he informed me, is not really necessary for a 3mm biopsy, which will typically easily heal on its own. Throughout the whole process, I looked at the counter in front of me and felt nothing at all.
Dr. W. placed gauze and a Band-Aid over the site. Because I was curious, he showed me the small chunk of tissue now floating in the 15mL tube. The skin punch had gone deep enough to reach a fatty layer of subcutaneous tissue – I was newly amazed that I had felt nothing. Then the tube was packaged away for shipment.
I was told to avoid exertion, such as heavy lifting, for the rest of the day that might stretch the biopsy site and tear the stitch. I was also given instructions for care of the site for the next two weeks: leave the bandage on for one day, and after that wash gently with soap and water twice a day, dry completely, apply Vaseline with a Q-tip to promote healing, and cover with a new bandage.
Leaving the office, I still felt a curious numb sensation in my hip, but otherwise felt completely normal. I then proceeded with my normal daily activities: walking around the lab all day, carrying groceries home, climbing steps. Also, kind of comically, I ended up moving some heavy boxes full of glassware and fruit flies for a friend who was setting up a new lab space, and needed a hand. Turns out, I’m bad at saying no. Happily, there were no adverse consequences to any of this. Though the numbness wore off within an hour, I never felt any pain associated with the biopsy site. I did get nervous when it came time, the next day, to change the Band Aid – without the input of a pain response, I felt like I had no idea what kind of carnage might be lurking underneath. To my relief, the wound was astonishingly small – a red dot about the size of a mustard seed – and I had barely bled. For the next two weeks, I was diligent about cleaning the site as instructed, and the wound began to heal. Within four weeks, it was a small, flat, slightly darkened circle of skin, not easy to distinguish from my many lovely moles and skin imperfections. I’m told that within a year, I probably won’t be able to discern the site, even if I look for it.
Dr. Zou has followed up with a few photos of my cells in culture – so far, as far as I know, they haven’t been transformed to iPS cells. But I’m excited to follow their progress and to follow the work that is done on these cells in the future. Especially since I work with iPSC in my day job, it’s neat to think of being part of a cutting-edge technology from a patient perspective as well. I love the thought that even when I’m not actively working on prion disease, my cells are out there contributing to work getting done. Readers of this previous post will already know me as a fanatical believer in research participation, and in seizing one’s opportunities to contribute — I would have donated cells to make iPSC even if I expected the procedure to be long, painful, or expensive. Instead, it was so easy that if something were to happen to the cells I’ve already donated, I’d give cells again without batting an eye.
To anyone considering donating skin cells for the creation of iPSC or any other research purpose, please feel free to contact me if you have any other questions. I’m happy to talk about the pros and cons of this experience and research participation generally. I genuinely think this was an incredible opportunity that I was lucky to have, and I’m excited to see what insights come of these resources in the years to come.

At the end of the day, tissue culture can be beautiful…

