When will treatments be available for prion disease?
This post is part of a series introducing the basics of prion disease. Read the full series here.
Running a research foundation devoted to finding a treatment for prion disease, the question we get most often is: are any experimental treatments available today? Unfortunately, right now the answer is that there’s nothing available for Creutzfeldt-Jakob disease or other human prion diseases. If you read our last post on what types of treatments could one day be available, you know that there are some good ideas in the pipeline – but they will take a while to be ready for patients. To understand why, it’s helpful to take a look at how new drugs are developed.
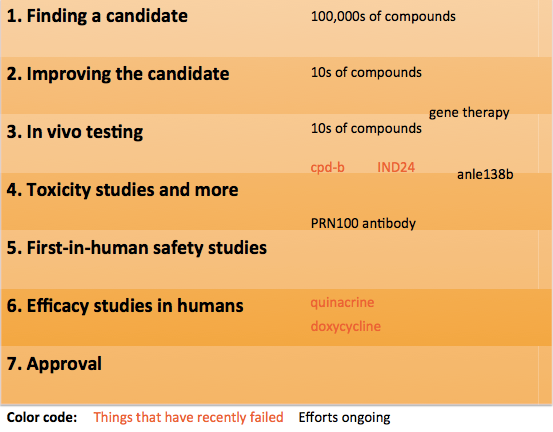
1. Finding a candidate
While there are a few different types of treatments under investigation, pretty much any treatment option is going to start out in a test tube. Small molecule drugs often come out of high-throughput screens, in which tens of thousands of chemical compounds are tested on small wells full of cells or purified proteins. Antibodies were conceived as a potential treatment after it was shown they could cure prion infections in cells in a dish. Gene silencing molecules have to be tested in cells to figure out which ones are effective enough to bother testing in animals, let alone humans.
A 96-well plate. In high-throughput screening, robotics are used to test hundreds of plates like these full of different chemical compounds.
2. Improving the candidate
As of today, hundreds of thousands of chemical compounds have been tested in high-throughput screening as potential drugs, and one or two dozen compounds have emerged as candidates worth pursuing further. From there, these compounds often undergo a few months to a few years of follow-up experiments to figure out how they work and make them work better, and to see whether they can get into the brain in high enough concentrations to do their job. In scientific and industry circles this step is often abbreviated ADME, because the four main properties you worry about with potential drugs are their absorption (do they get into the body?), distribution (do they get to the right tissues within the body?), metabolism (how are they broken down?), and excretion (how are the breakdown products disposed of?).
3. In vivo testing
The compounds that perform well on the above measures will eventually be tested for efficacy in animal models, usually mice. In some cases, people will do step 3 first to see if a compound works at all, and then go back to step 2 and try to make it even better. But in either case, one animal experiment can take several months. To really canvass whether the compound works in a variety of animal models, against different forms of prion disease, at different doses and different timepoints during disease can easily take a year or two.
4. Toxicity studies and more
If a potential drug looks very promising up through step 3, it will usually go on to a very rigorous battery of toxicity tests in a laboratory certified as using Good Laboratory Practices. In the industry, this step is often abbreviated “GLP Tox”. It takes a lot of data from animals to see whether a treatment looks safe enough to be tested in humans. To make sure people don’t fudge the numbers, drugs almost always need to have been tested under “GLP” conditions before they can get to a clinical trial.
In parallel with GLP Tox, anyone developing a new drug will also need to figure out how they’re going to manufacture so that it’s pure enough to give to humans, and how they’ll design a clinical study so as to minimize risk to the people participating.
5. First-in-human safety studies
A potential treatment that’s gotten through steps 1-4 with passing grades is ready to apply for Investigational New Drug (IND) status under the FDA in the United States, or equivalents in many other countries. IND status means that a drug can now be tested for safety in humans under the tightly controlled and closely monitored conditions of a Phase I Clinical Trial. A Phase I trial only seeks to test the safety of a potential drug. If the drug is expected to be pretty safe based on the animal data, the Phase I trial will often use healthy volunteers – adventurous souls who, for a couple thousand dollars, will take a molecule no human has ever taken before, and then be watched carefully to make sure they’re okay.
Sometimes, instead of healthy volunteers, safety studies will be done directly in patients with the disease of interest. This happens sometimes with drug candidates that appear perfectly safe, but is especially important if there are concerns about toxicity based on animal studies. In very deadly conditions like metastatic cancer or, hypothetically, prion disease, a potential drug with some serious toxicity can sometimes still make it to the IND stage if its efficacy appears to outweigh its toxicity. For such drug candidates, testing in patients is essential, as it wouldn’t be ethical to give the drug to healthy volunteers.
6. Efficacy trials in humans
If a drug appears safe in Phase I, it can be given to patients for long enough to see if it helps them, in Phase II and Phase III Clinical Trials. “Helps them” can mean a lot of different things depending upon the clinical trial. Before starting the trial, the scientists and/or drug company running the trial need to declare what the “endpoints” of the trial will be – what things they’ll measure to see if the trial was a success. For instance, in two recently-reported clinical trials for prion disease, of quinacrine [Geschwind 2013] and doxycycline [Haik & Marcon 2014], the endpoint was simply survival: did treated patients live longer than untreated patients? In the proposed clinical trial of PRN100, the endpoint would be a rating scale of patients’ functional capacity: do treated patients retain more cognitive function than untreated patients? Clinical trials can use plenty of other endpoints too – for example, a particular “biomarker” such as a patient’s performance on a cognitive or motor test, or some measurement of their brain’s appearance on an MRI scan, could be endpoints. So far, no specific biomarkers have been proposed as endpoints in prion disease.
There are a few different designs for Phase II and III Clinical Trials testing a potential drug’s efficacy. The gold standard is a randomized placebo-controlled double-blind trial: half the patients get placebo and half get the real drug, and neither the patients nor the doctors treating them know which is which. Success in such a trial means a lot, as many sources of bias can be ruled out. A disadvantage is that not all patients get the drug – and for rare diseases, that means it’s harder to find enough patients. Some clinical trials are “open label”, meaning that patients (or their families, on their behalf) can choose whether to take a drug, and the treated patients’ endpoints are compared to historical data on other patients. The risk in this type of trial is that a drug that doesn’t work might appear effective just because the healthier patients are the ones who choose the drug. For instance, both quinacrine and doxycycline appeared to be effective because patients that took them lived longer than patients that didn’t [Geschwind 2013, Tagliavini 2008, Ponto 2013], but ultimately did not appear effective in a randomized placebo-controlled double-blind trial [Geschwind 2013, Haik & Marcon 2014].
7. Approval
If a drug can make it through all these steps and seems safe and effective, then it’s time to apply to FDA – or the equivalent in other countries – for approval to sell the drug to patients. Few drug candidates make it this far, and you often hear scary estimates of how much it costs (ten billion dollars!) and how long it takes (15 years!). We’ll talk more about this in a minute.
Where are we now?
First, let’s take a look at where potential drugs for prion disease are in this process. Here’s a quick diagram to show a bit of what’s been happening recently:
At the top of the ladder, several different groups worldwide are working on high-throughput screening to find new small molecule drugs against prion disease. Lately, every few months we learn about new studies in which tens or hundreds of thousands of compounds were tested. Only a few tens of these compounds will look good enough to make it to steps 2 and 3.
Recently two notable compounds have failed in step 3: cpd-b and IND24. These both looked really good in early animal studies with mouse prion strains, turned out not to work against human prion strains [Berry 2013, Lu & Giles 2013]. That’s disappointing, but the discovery of compounds that even work against mouse prions is still a really important proof-of-concept which demonstrates that it’s feasible to use small molecule drugs to treat prion disease, and the lesson that some compounds might be strain-specific is an important one to have learned.
Other approaches are in the pipeline too – for instance, a variety of gene therapy approaches are in various combinations of Step 2 and 3. Anle138b is in Steps 3 and 4 – it succeeded against one mouse prion strain and is currently being tested against other prion strains, while it also recently got an investment to carry it through Step 4.
MRC Prion Unit’s PRN100 antibody has apparently completed all of its preclinical development and the possibility of a first-in-human safety study is being discussed.
Finally, as mentioned above, two already-approved drugs, quinacrine and doxycycline, were able to skip all the preliminary steps and go straight to Step 6, where they appear to have failed, though one clinical trial of doxycycline is still ongoing.
So when will new drugs be available?
The true answer is that we don’t know. It’s good to be mentally prepared for drug discovery to be a many-year-long process, but on the other hand, one shouldn’t take those estimates of, say, 15 years as being a minimum. The truth is, drugs don’t usually fail just because costs are high and regulations are tough – they fail because they don’t work! When we find something that does work, which is what matters, it will be quicker.
And as you see from the above diagram, it is not as though we are standing empty-handed at the beginning of the process. Many things have been tried and failed in prion disease, and we’ve learned a lot from all those failures. Other things are still being tried and may yet work.
Finally, it’s not necessarily the case that patients can’t get a drug until it’s approved. Patients who participate in clinical trials get drugs much sooner, and in some cases, once a drug has received IND status, Expanded Access can let some patients receive the drug who don’t qualify to enter the clinical trial. Note that this option is not available today for any potential drugs for prion disease. We receive frequent inquiries about anle138b, and we’re told that MRC Prion Unit receives frequent inquiries about PRN100, however, neither of these treatments has achieved IND status at this time.
conclusion
No one can predict the future, and we don’t know better than anyone else does when exactly a new treatment for prion disease will reach people who need it. But the two most important take-home messages are that: 1) there are many possible treatments at various stages in the pipeline, 2) we’ve learned from things that have been tried and failed, so we’re not at the very beginning of this process.
In addition, if you’ve read the rest of our FAQ, you may have noticed one other big advantage that we have in looking for a treatment for prion disease: the fundamental biology of prions is really well understood, more so than the biology of many other diseases. That’s one of many reasons why, though there are no treatments available today, we are very optimistic about the long-term prospects for treating prion disease.